This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.
Related Articles
00 / 00

This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.
Assistant Professor
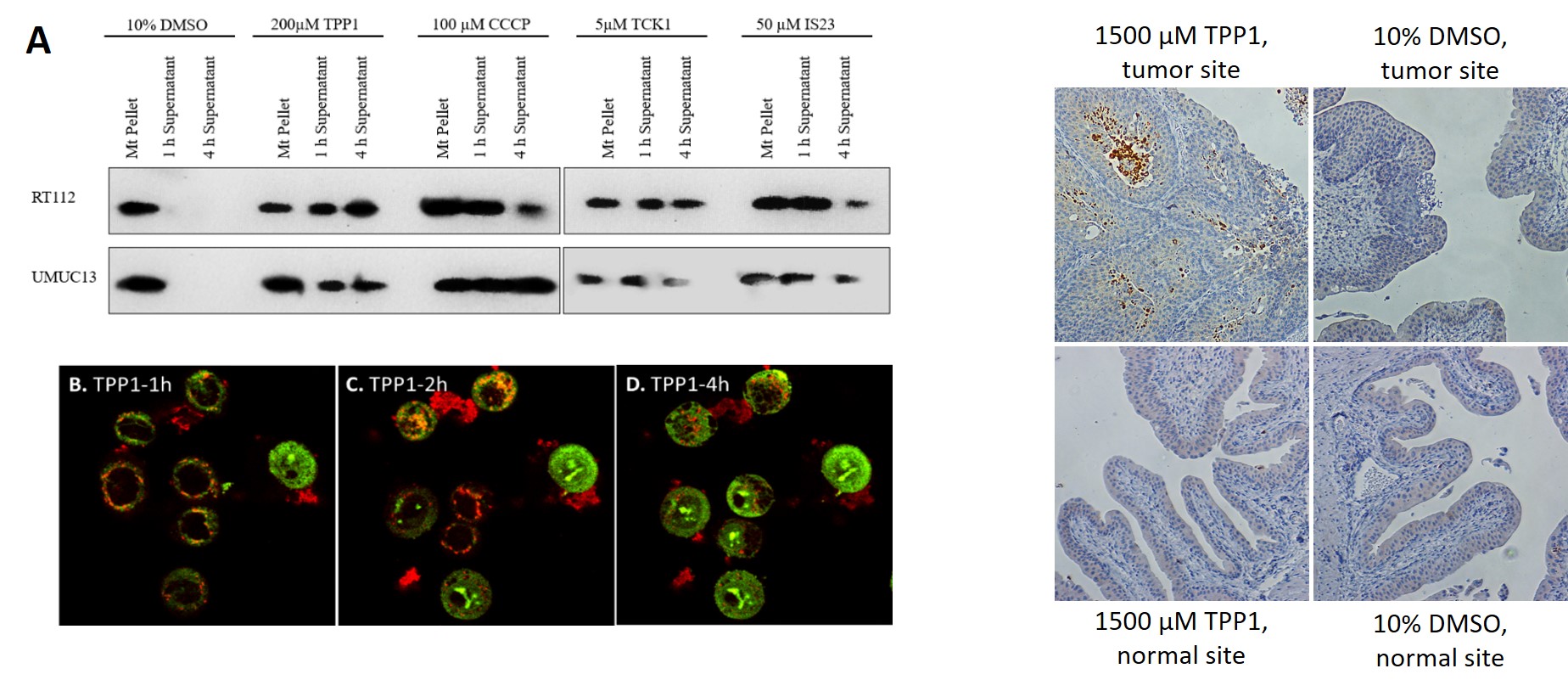


The Abbosh Lab is focused on translational research in bladder cancer. The lab has multiple projects which all focus on achieving and identifying the pT0 state in patients with localized (curable) bladder cancers. These projects include novel diagnostic tests to detect residual bladder cancer after neoadjuvant therapy, novel mouse models on which to develop the basis for personalized medicine trials, annotation and experimental manipulation of the human bladder and bladder cancer microbiome, annotation and experimental manipulation of immune response to chemotherapy for patients with bladder cancer, and development of novel intravesical therapies to replace BCG. The lab uses human-derived biological fluids and tissues, cell lines, and mouse models to perform these studies. These studies are all intended for clinical translation.
When patients have muscle-invasive bladder cancer, the standard of care is treatment with cisplatin-based chemotherapy before surgery to remove the entire bladder. This necessitates a significant life change since most often the urine will be diverted to a cutaneous ostomy postoperatively. At the time of bladder removal, about 30-40% of patients will not have residual cancer left in their bladder. Patients and doctors alike wonder if bladder removal is necessary if there is no tumor left after chemotherapy. The answer at this point in time is “yes” because doctors do not currently have a way to reliably identify patients who will respond to chemotherapy (prospectively), or patients who actually did respond to chemotherapy (retrospectively). We are currently using next generation sequencing of liquid biopsy samples to prospectively and retrospectively identify responders, with the long term goal of safe radical cystectomy avoidance in the future.
Bladder cancer sequencing efforts have revealed a large number of potentially therapeutically tractable targets, but how to target them is still unknown. These genetic alterations can be modeled in mice using the exciting new genome editing tool CRISPR. Using this technology, we are making many new bladder cancer models by introducing combinations of mutations into the murine urothelium to try to model the human disease more accurately in mice. This will hopefully allow us to marry individual mutations to therapeutic strategies in anticipation of personalized medicine algorithms in humans.
Neither the urine nor the bladder are sterile environments. This has been increasingly recognized over the last 10 years using new approaches to detect bacteria, fungi, and viruses. The bladder cancer microbiome is only just now being discovered and studies to annotate the types of organisms present in the bladder and urine from patients with and without bladder cancer are not fully described. Using mostly marker gene metagenomics, the Abbosh lab is now cataloguing the types of organisms present in the bladder with the long term goal to determine how the microbes affect the tumor microenvironment and possibly how they alter the response to medical therapies (for better or worse).
In collaboration with the laboratory of Dr. Wiley Youngs (University of Akron), we also have developed a series of imidazolium-based compounds that briskly and quickly induce apoptosis through a novel mechanism which unequivocally targets the mitochondria. When instilled into to the murine bladder, they do not cause histopathological effect to normal tissues even at high doses but do induce apoptosis in established tumors. Since publishing the first-generation compound, we have developed more potent compounds and are working towards identifying the mitochondrial drug receptor. See figure.
The Abbosh Lab also studies kidney cancer. PBRM1 and SETD2 are commonly mutated in kidney cancer. Despite their frequency, little is known about role they play in the genesis of kidney cancer. We are currently exploring changes that occur after their loss and identifying potential avenues for personalized treatment strategies in advanced cases of kidney cancer.
Braun A.,Abbosh P.H., Muscle-invasive bladder cancer arising after prostate radiotherapy: A rare entity with unique genomic features. Eur Urol. 81(5): 474-475, 2022. https://www.ncbi.nlm.nih.gov/pubmed/35109971.
Sikder R.K., Ellithi M., Uzzo R.N., Weader D.J., Metz A.L., Behbahani A., McKenzie E.R., El-Deiry W.S.,Abbosh P.H., Differential effects of clinically relevant n- versus c-terminal truncating cdkn1a mutations on cisplatin sensitivity in bladder cancer. Mol Cancer Res. 19(3): 403-413, 2021. PMC7925368 https://www.ncbi.nlm.nih.gov/pubmed/33272936
Stromyer M.L., Southerland M.R., Satyal U., Sikder R.K., Weader D.J., Baughman J.A., Youngs W.J., Abbosh P.H., Synthesis, characterization, and biological activity of a triphenylphosphonium-containing imidazolium salt against select bladder cancer cell lines. Eur J Med Chem. 185: 111832, 2020. 4.833 PubMed
Li Q, Damish AW, Frazier Z, Liu D, Reznichenko E, Kamburov A, Bell A, Zhao H, Jordan EJ, Gao SP, Ma J, Abbosh PH, Bellmunt J, Plimack ER, Lazaro JB, Solit DB, Bajorin D, Rosenberg JE, D'Andrea AD, Riaz N, Van Allen EM, Iyer G, Mouw KW, ERCC2 Helicase Domain Mutations Confer Nucleotide Excision Repair Deficiency and Drive Cisplatin Sensitivity in Muscle-Invasive Bladder Cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2019; 25(3):977-988. NIHMSID: NIHMS1016538 PubMed [journal] PMID: 29980530, PMCID: PMC6434536
Abbosh P., Sundararajan S., Millis S.Z., Hauben A., Reddy S., Geynisman D.M.,Uzzo R., Molecular and genomic profiling to identify actionable targets in chromophobe renal cell cancer. Eur Urol Focus. 4(6): 969-971, 2018. PubMed
Geynisman D.M., Abbosh P.H., Plimack E.R.,Zibelman M., Chemoimmunotherapy in metastatic urothelial carcinoma. Eur Urol. 73(5): 760-762, 2018. Editorial. 17.298
Hernandez Borrero L.J., Sikder R., Lulla A., Gokare P., Del Valle P.R., Tian X., Zhang S., Abbosh P.H.,El-Deiry W.S., Bcl-2 protein targeting by the p53/p21 complex-letter. Cancer Res. 78(10): 2770-2771, 2018. Letter. 8.378
Liu D., Abbosh P., Keliher D., Reardon B., Miao D., Mouw K., Weiner-Taylor A., Wankowicz S., Han G., Teo M.Y., Cipolla C., Kim J., Iyer G., Al-Ahmadie H., Dulaimi E., Chen D.Y.T., Alpaugh R.K., Hoffman-Censits J., Garraway L.A., Getz G., Carter S.L., Bellmunt J., Plimack E.R., Rosenberg J.E.,Van Allen E.M., Mutational patterns in chemotherapy resistant muscle-invasive bladder cancer. Nat Commun. 8(1): 2193, 2017. PMC5736752. 11.878 PMCID: PMC5736752
McIntosh A.G., Li T., Ito T., Mannion J., Dziemianowicz M., Waingankar N., Haseebuddin M., Chen D.Y.T., Greenberg R.E., Viterbo R., Kutikov A., Uzzo R.G., Smaldone M.C.,Abbosh P.H., Wbc associates with readmission following cystectomy. Bladder Cancer. 3(2): 95-103, 2017. PMC5409152.
This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.












