Precision Medicine at Fox Chase Cancer Center dates back to Hungerford’s discovery of the Philadelphia Chromosome, Daly’s creation of one of the first cancer family registries in the US, Knudson’s retinoblastoma two-hit hypothesis for tumor suppressor genes, and gene discoveries that predict risk such as Testa’s recent BAP1 discovery as a susceptibility gene for familial mesothelioma, uveal melanoma, and other tumors.
Efforts in the past decade have involved the development of in-house capabilities for genomic sequencing including Cancer-Code used in clinical practice. Additionally in the past five years there has been a broad range of precision medicine activities including identification of genomic biomarkers for bladder cancer response to chemotherapy, efforts to profile neuroendocrine cancers, and metastatic lesions in approximately 7000 colorectal cancers through our collaboration with Caris Life Sciences.
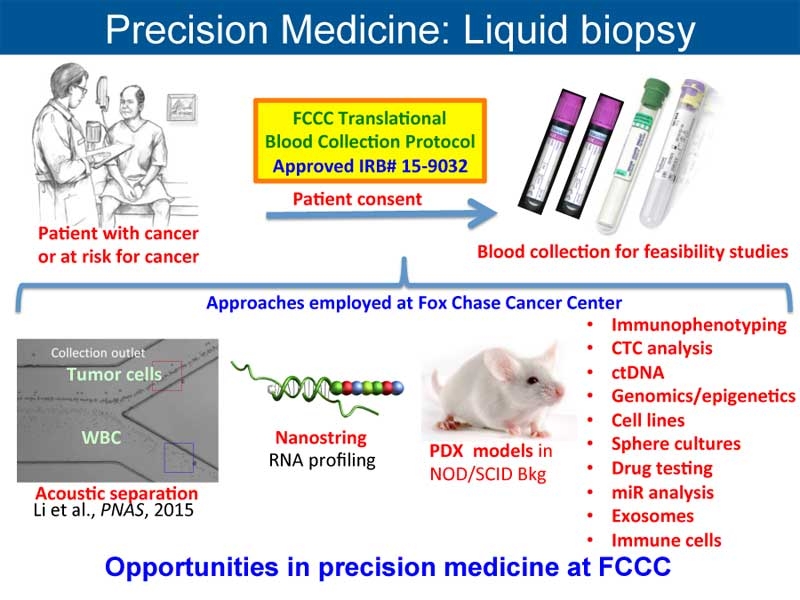
The goal of the precision medicine effort at Fox Chase Cancer Center is to investigate and direct relevant personalized interventions that impact on the health care of patients with cancer as well as those at increased risk for cancer. Several activities, both new and ongoing, are focused on those goals.
- There continues to be a focus on understanding and communication of genomic medicine with respect to patients, genetic counselors, or physicians. The Risk Assessment Program is a unique strength at FCCC in support of precision medicine.
- The Precision Medicine Steering Committee (PMSC) meets regularly to discuss the status and vision for precision medicine efforts at FCCC. The Committee includes broad representation from various stake-holders both clinicians, research investigators and the administration.
- The development of translational research in precision medicine is catalyzed through the Translational Research Disease Groups as a mechanism to support precision medicine translational research
- Fox Chase is a participant in the national precision medicine clinical trial (called MATCH) that is enrolling patients into therapeutic protocol arms based on actionable gene mutations. Lori Goldstein serves on the ECOG committee for this protocol and Ranee Mehra is the co-chair for the ALK+ arm of the national trial.