
PHILADELPHIA (October 23, 2025) — In a new study published in the prestigious journal Nature, researchers at Fox Chase Cancer Center have uncovered a new mechanism by which the body’s immune system responds to viral infections, a finding that can potentially be harnessed to attack cancer.
The study focuses on a protein called ZBP1, long known as a cellular “sensor” for viral invaders. Traditionally, scientists believed ZBP1 detected foreign genetic material from viruses, triggering infected cells to self-destruct and prevent the spread of infection. However, the new research reveals that the signal ZBP1 detects doesn’t only come from the virus but also from the host cell itself.
New Directions for Medicine
“This finding turns a fundamental assumption of immunology on its head,” said Siddharth Balachandran, PhD, Director of the Center for Immunology at Fox Chase and senior author on the study. “We used to think viruses provided the signals that alert cells to danger. Now we know our own cells can create those signals, and that revelation opens entirely new directions for medicine.”
Balachandran, whose lab has spent years decoding how viruses and host cells engage in this molecular tug-of-war, conducted the study with an international team of researchers and lead author Chaoran Yin, PhD, an Assistant Research Professor in his lab.
A Self-Made Distress Signal
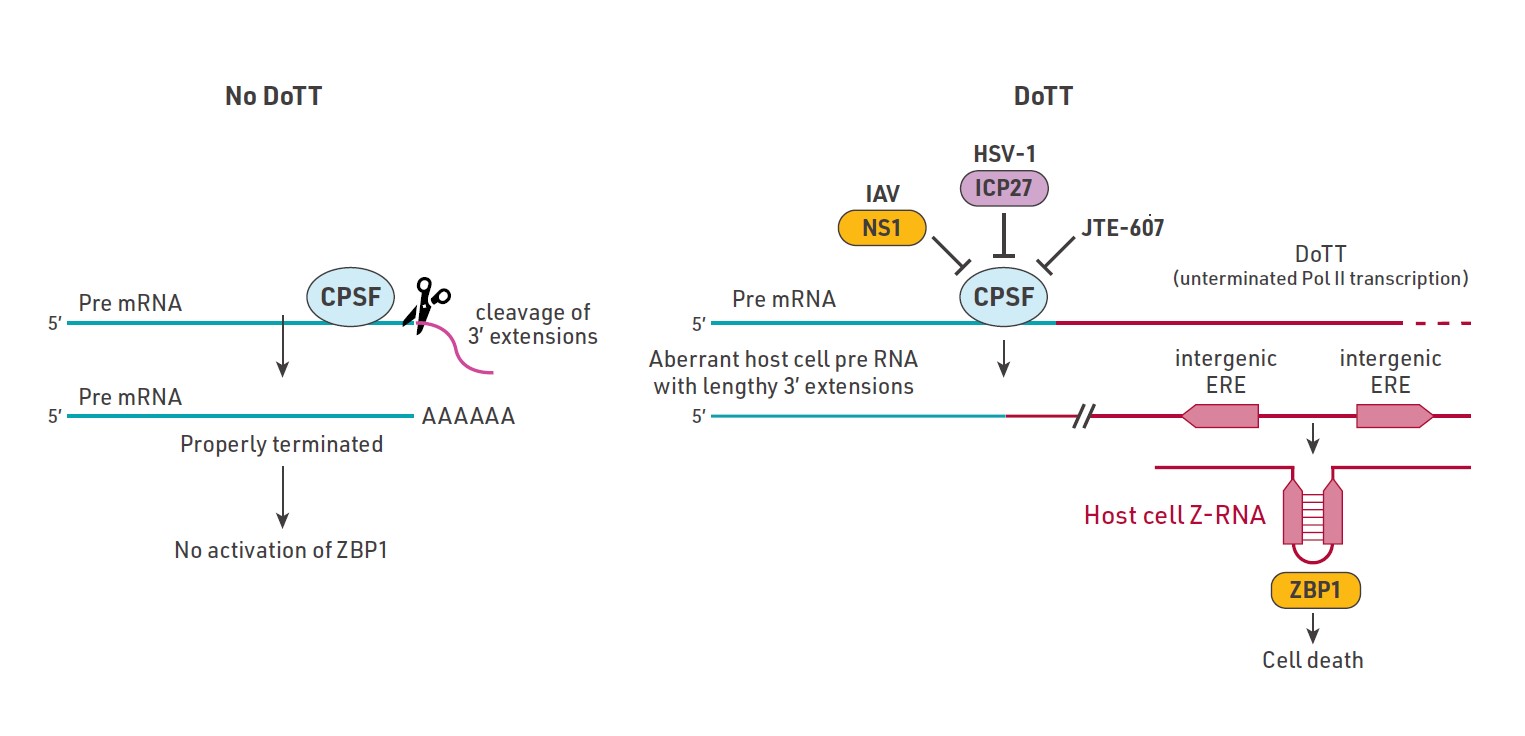
Instead of sensing something made by viruses, infected cells produce their own unusual form of RNA, called Z-RNA, as a distress signal. This self-made RNA sets off a chain reaction that leads infected cells to self-destruct before viruses can highjack them.
Researchers have also traced these Z-RNAs back to endogenous retroelements, which are inactive viral remnants once dismissed as “junk” DNA that have integrated into the genome throughout evolution. The findings show that the host, not the pathogen, generates the crucial trigger for antiviral defense.
Possible Cancer Therapy Applications
This discovery not only advances the basic science of viral immunity, but it also carries significant implications for cancer immunotherapy, a type of treatment that uses the body’s immune system to recognize, attack, and kill cancer cells. Using molecular and biochemical methods, the team found that the same cellular pathways that generate host Z-RNA during viral infection can be chemically activated inside tumors.
By reawakening the cell’s own retroviral elements, researchers can make tumors “look infected,” Balachandran said, prompting the immune system to attack them.
“The immune system evolved to fight microbes, not cancer,” Balachandran explained. “But if we can make a tumor mimic a viral infection, we can trick the immune system into seeing it as dangerous.”
Foundational Virus Research
The study builds on previous research done by Balachandran’s lab that showed
how influenza viruses kill the cells they infect. The research team determined that influenza viral infection triggers a specialized form of programmed cell death, necroptosis, which is a key process in controlling viral spread.
This work defined how influenza functions as a lytic virus, a type of virus that reproduces by hijacking a host cell, using it to create many copies of itself, and then bursting the cell to release the new viruses.
This finding revealed that cell death during infection is not random damage but a coordinated, immune-regulated event. The team furthered this research in another paper where they uncovered the mechanism of this cell death, showing that the protein ZBP1 acts as a sensor that detects when a cell has been infected. This was the first major insight into how a cell knows it’s infected and why it decides to die, connecting ZBP1 activity to disease severity and inflammation.
Then, in a paper published in the journal Cell in 2020, they determined how ZBP1 was activated, implicating Z-RNA generation as the initiating event in this process. This set the stage for the current study, which reveals that these Z-RNAs, previously assumed to be of viral origin, are actually produced by the infected cell itself.
Reprogramming the Immune System
Balachandran’s lab is now partnering with the Molecular Modeling Facility at Fox Chase to design a new class of small molecules that safely trigger these antiviral pathways in cancer cells. The goal is to expand immunotherapy effectiveness to cancers that currently don’t respond to it.
“We’re now taking what we’ve learned from viruses and using it to reprogram how the immune system sees cancer,” said Balachandran.