Weather Alert: Following the winter storm, all Temple Health hospitals, campuses and clinical locations remain open. Patients will be contacted directly if their visit is affected. Please check TempleHealth.org or FoxChase.org for updates and monitor myTempleHealth for changes to scheduled appointments.
Breadcrumb
- Home
- Kyoko Hayakawa
Kyoko Hayakawa, MD, PhD

Emeritus
Lab Overview
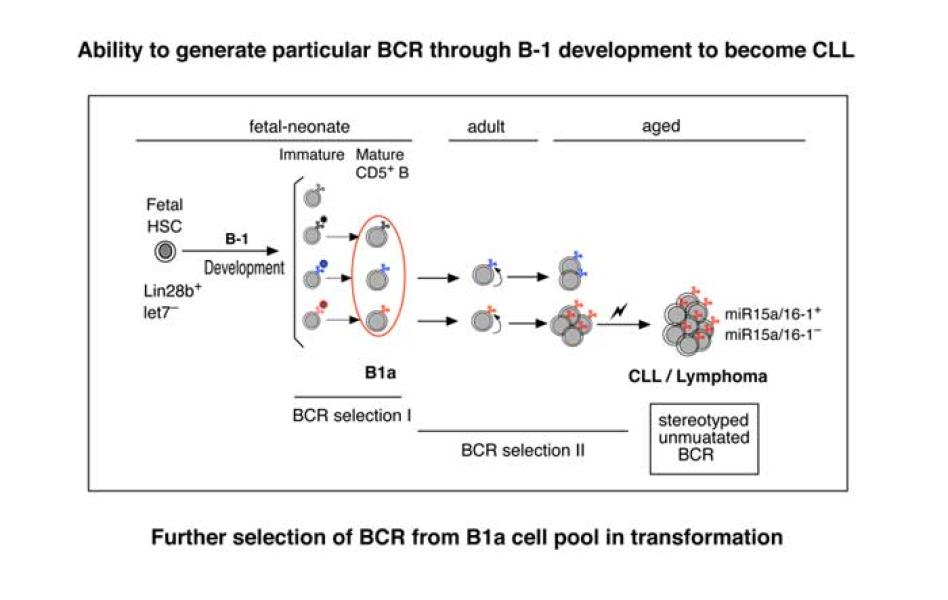
B1 B cells generated early in life by fetal/neonatal B-1 development in mice include autoreactive cells with detectable CD5 upregulation induced by BCR signaling. Part of such early-generated B1 B cells are maintained by self-renewal for life, continuously providing auto- and poly-reactive antibodies, serving a protective role. However, the B1 B cell subset has a higher risk of dysregulated growth and progression to chronic lymphocytic leukemia (CLL)/lymphoma during aging, than other B cell subsets. We have generated several autoreactive germline gene BCR models that enable comparison of B cells generated under conditions of natural exposure to autoantigen for such studies. Analysis of these mice has been key in understanding the importance of BCR and BCR signaling for generation of different B cell subsets and in investigating the cellular origin of CLL. Early generated B1 B cells can circulate, and are constantly exposed to the microenvironment, promoting life-long self-renewal. Thus, in addition to the importance of B cell origin, BCR signaling, and genetic background influences, the role of the microenvironment is another important issue for understanding how B1 cells progress to become CLL.
Educational Background
- Research Fellow, Institute for Molecular and Cellular Biology, Osaka University, Osaka, Japan (1984-1987)
- Postdoctoral Fellow, Stanford University School of Medicine, Department of Genetics,Palo Alto, CA (1980-1984)
- Postdoctoral Fellow, Department of Immunology, University of Tokyo, School of Medicine Tokyo, Japan (1978-1982)
- PhD, Immunology, Chiba University, 1978
- MD, Medicine, Fukushima Medical University, 1974
Certifications
- Japanese Medical License (May 28, 1974)
Memberships
- Faculty of 1000 biology
Honors & Awards
- Arthritis Foundation (1993-1996)
- Fellow of Sankyo foundation of Life Science (1985-1987)
- Fellow of Japan society for the Promotion of Science (1978-1979)
People
Research Interests
Natural autoreactive B cell development and chronic leukemia
- Natural autoreactive B cell generation by fetal/neonatal B cell development (B-1).
- B cells generated by B-1 cell development have the highest propensity to become chronic leukemia/lymphoma (CLL) in mice.
- A mouse CLL with a defined anti-non-muscle myosin IIA autoreactive BCR (B cell antigen receptor) resembles aggressive human CLL.
Selected Publications
Hayakawa K, Formica AM, Brill-Dashoff J, Shinton SA, Ichikawa D, Zhou Y, Morse III HC, and Hardy RR. Early generated B1 B cells with restricted BCRs become chronic lymphocytic leukemia with continued c-Myc and low Bmf expression. J. Exp. Med. 213:3007-3024, 2016. PubMed
Hayakawa K, Formica AM, Colombo MJ, Shinton SA, Brill-Dashoff J, Li YS, and Hardy RR. Loss of a chromosomal region with synteny to human 13q14 occurs in mouse chronic lymphocytic leukemia that originates from early-generated B-1 B cells. Leukemia 30:1510-1519, 2016. PubMed
Ichikawa, D., Asano, A., Shinton, S.A., Brill-Dashoff, J., Formica, A.M., Velcich, A., Hardy, R.R., and Hayakawa K. Natural anti-intestinal goblet cell autoantibody production from Marginal zone B cells. J. Immunol. 194:606-614, 2015 PMID: 25480561 PMCID: PMC4282382 PubMed
Zhou, Y., Li, Y.S., Rao Bandi, S., Tang, L., Shinton, S.A., Hayakawa, K., and Hardy, R.R. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J. Exp. Med. 212:569-80, 2015. PubMed
Wen, L., Brill-Dashoff, J., Shinton, S.A., Asano, M., Hardy, R.R., and Hayakawa, K. Evidence of marginal zone B cell positive selection in spleen. Immunity 23:297-308, 2005. PubMed
Wen, L., Shinton, S.A., Hardy, R.R., and Hayakawa, K. Associaiton of B-1 B cells with follicular dendritic cells in spleen. J. Immunol. 174:6918- 6926, 2005. PubMed
Hayakawa, K., M. Asano, S. A. Shinton, M. Gui, M., L.-J. Wen, J. Dashoff, and Hardy, R. R. Positive selection of anti-Thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J. Exp. Med. 197:87-99, 2003. PubMed
Hayakawa, K., M. Asano, S. A. Shinton, M. Gui, D. Allman, C.L. Stewart, J. Silver, and R. R. Hardy. Positive selection of natural autoreactive B cells. Science 285:113-116, 1999. PubMed