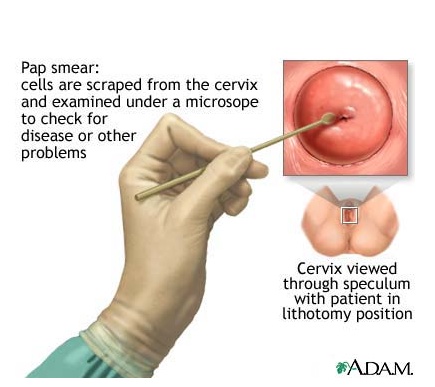
The End of the Pap Test for Cervical Cancer?
-
For decades, the Pap test had been the only test available to screen for cervical cancer. The Pap is simple, safe, inexpensive, and most importantly, very effective. The number of cases of cervical cancer in the US has dropped by more than half over the past 30 years, mostly thanks to Pap testing. But, unfortunately, despite widespread availability of Pap tests, about 12,000 women each year are still diagnosed with cervical cancer.
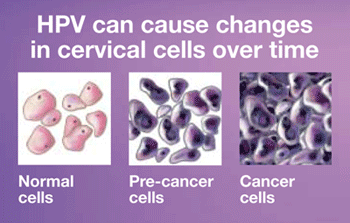
Certain strains of human papilloma virus (HPV) cause nearly all cases of cervical cancer, and guidelines for cervical cancer screening have been evolving to incorporate HPV testing. The American Cancer Society’s latest guidelines recommend a Pap every 3 years for women between the ages of 21 and 29. For women 30 and older, either a combined Pap and HPV test can be performed every five years (so called “contesting”), or a Pap alone can be performed every three years. When a Pap test is inconclusive, an HPV test may be used to help determine if further evaluation by colposcopy is warranted. Under current recommendations, women in their 20’s should not have HPV testing because positive tests are common and may lead to unnecessary procedures, despite the fact that most infections will resolve without any treatment.
On April 24, 2014, the drug maker Roche has received FDA approval for the cobas® HPV Test test to become a first choice option, instead of Pap testing, for cervical cancer screening in women aged 25 and older. With this approval, the FDA is indicating that the studies of the cobas HPV test show it to be both safe and effective as a first step for cervical cancer screening. The cobas HPV test checks for HPV types 16 and 18, which together are responsible for about 70% of cervical cancers, as well as 12 other high-risk HPV types.
Most sexually active young people will contract HPV, and only very rarely does it persist to cause cancer. In most individuals, the immune system will clear the infection within several months, which is why the current guidelines do not encourage HPV testing in women under the age of 30. These previous recommendations are in conflict with the FDA’s approval of the cobas HPV test for women starting at age 25, and further research might be necessary to decide what is best for women between the ages of 25 and 30.
A number of women’s groups including the American Medical Women’s Association and Our Bodies Ourselves have come out against replacing the tried and true (as well as less expensive) Pap test with the HPV test alone, warning that that change would be a “radical shift” in current medical practice that could lead to confusion, overtreatment, and higher costs. Seventeen patient advocacy groups, including Consumer Union, the Cancer Prevention and Treatment Fund, and the National Alliance for Hispanic Health, signed a letter to the FDA, stating that the proposed HPV test would be replacing “a safe and effective well-established screening tool and regimen that has prevented cervical cancer successfully in the US with a new tool and regimen not proven to work in a large US population.”
It is important to know that while the Pap is a great and effective test for detecting cervical precancer and cancer, it is not perfect. The search for better screening methods is ongoing. The approval of the cobas HPV test means doctors have yet another tool to help improve our detection of these problems. FDA approval does not mean that the HPV test must or should be used instead of a Pap test, only that studies have shown it to be a safe and effective option for screening. Approval of this HPV test for primary screening does NOT change the current recommendations for when and how women should be tested. In addition, guidelines by the major organizations concerned with cervical cancer screening still need to be developed as to how patients with a positive HPV test should be followed up, though the FDA has stated that women who have an HPV test positive for types 16 or 18 (the highest risk types) should undergo colposcopy (a special magnified exam of the cervix with biopsies) as the next step, while women who test positive for one of the other 12 high-risk strains should have a Pap test to help decide whether colposcopy is necessary.
The Pap test isn’t going away. Women should discuss their options for screening with their doctors, and all parents should have their daughters and sons vaccinated against the HPV virus. The vaccine is safe and effective, and is probably the best method we have to prevent cervical cancer.
