Related Articles
00 / 00
Transit Alert: SEPTA is cutting bus, subway, and rail service across Philadelphia. These changes may affect patients, visitors, and staff traveling to and from Fox Chase Cancer Center locations. Read full update.

This Fox Chase professor participates in the Undergraduate Summer Research Fellowship.
Learn more about Research Volunteering.
The process of neoplastic transformation can be conceptually divided into two components. The first of these, proliferative transformation, refers to the ability of transformed cells to bypass growth suppression signals, suriviving and dividing when normal cells would not. The second, morphologic transformation, refers to loss of normal cytoskeletal architecture, often accompanied by decreased adhesion and acquisition of the ability to invade surrounding tissues. These two fundamental properties are intimately linked to one another, although experimentally they can be dissected apart through the use of gain-of-function and loss-of-function manupulation of signaling molecules. The overall focus this research is in uncovering the roles of protein phosphorylation in governing these two fundamental aspects of cancer biology.
Chernoff has trained dozens of postdoctoral and graduate students at Fox Chase. He serves as an adjunct professor for the Drexel University School of Medicine and for the University of Pennsylvania, and is a member of the Cancer Signaling and Epigenetics research program at Fox Chase.
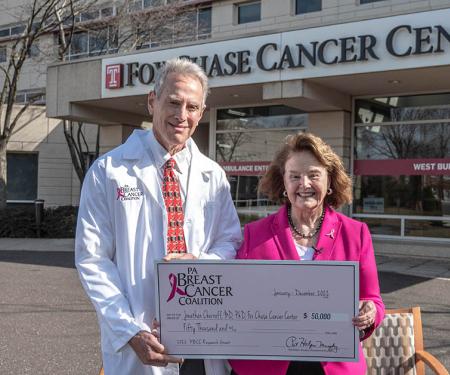
In addition to these roles, Chernoff has served on multiple external advisory boards, including the Scientific Advisory Board for Nexgenix Pharmaceuticals, as well as advisory boards for Genentech, Novartis Pharmaceuticals, and the New York University (Perlmutter) Cancer Center, among others. He has also taken on roles for multiple committees and programs within Fox Chase.
Chernoff earned his bachelor’s degree in molecular biophysics and biochemistry from Yale College and his medical degree and doctorate from the Mount Sinai School of Medicine in New York. He completed his residency in internal medicine at the University Health Center of Pittsburgh and a clinical fellowship in medical oncology at Johns Hopkins Oncology Center. He then held a postdoctoral fellowship in cellular and developmental biology at Harvard University before coming to Fox Chase. Collapse


The process of neoplastic transformation can be conceptually divided into two components. The first of these, proliferative transformation, refers to the ability of transformed cells to bypass growth suppression signals, surviving and dividing when normal cells would not. The second, morphologic transformation, refers to loss of normal cytoskeletal architecture, often accompanied by decreased adhesion and acquisition of the ability to invade surrounding tissues. These two fundamental properties are intimately linked to one another, although experimentally they can be dissected apart through the use of gain-of-function and loss-of-function manipulation of signaling molecules. The overall focus this research is in uncovering the roles of key protein kinases that govern these two fundamental aspects of cancer biology.
Karchugina S., Chernoff J., Detection of heterodimerization of protein isoforms using an in situ proximity ligation assay. J Vis Exp, (140)2018. PMC6235567. 1.108
Kim S.M., Nguyen T.T., Ravi A., Kubiniok P., Finicle B.T., Jayashankar V., Malacrida L., Hou J., Robertson J., Gao D., Chernoff J., Digman M.A., Potma E.O., Tromberg B.J., Thibault P.,Edinger A.L., Pten deficiency and ampk activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 8(7): 866-883, 2018. PMC6030497. 26.370
Stepanova D.S., Braun L., Chernoff J., A new concept in nf2 pharmacotherapy: Targeting fatty acid synthesis. Oncoscience. 5(5-6): 126-127, 2018. PMC6049319.
Prudnikova T.Y., Chernoff J., The group i pak inhibitor frax-1036 sensitizes 11q13-amplified ovarian cancer cells to the cytotoxic effects of rottlerin. Small GTPases. 8(4): 193-198, 2017. PMC5680705. 2.048
Semenova G., Chernoff J., Targeting pak1. Biochem Soc Trans. 45(1): 79-88, 2017. PMC5973817. 4.291
Semenova G., Stepanova D., Deyev S.M., Chernoff J., Medium throughput biochemical compound screening identifies novel agents for pharmacotherapy of neurofibromatosis type i. Biochimie. 135: 1-5, 2017. PMC5405558. 3.362
Araiza-Olivera D, Feng Y, Semenova G, Prudnikova TY, Rhodes J, Chernoff J. Suppression of RAC1-driven malignant melanoma by group A PAK inhibitors. Oncogene, 37(7):944-52, 2018. PMC5814328 Collapse
Semenova G, Stepanova DS, Dubyk C, Handorf E, Deyev SM, Lazar AJ, Chernoff J. Targeting group I p21-activated kinases to control malignant peripheral nerve sheath tumor growth and metastasis. Oncogene, 36(38):5421-31, 2017. PMC5608634